NUWIQ is different from rFVIII products made from hamster cell lines
FVIII Innovation at Octapharma
How NUWIQ is made
No Hamster Cell Lines, No Animal- or Human-Proteins, and No Human Albumin Used to Make NUWIQ1
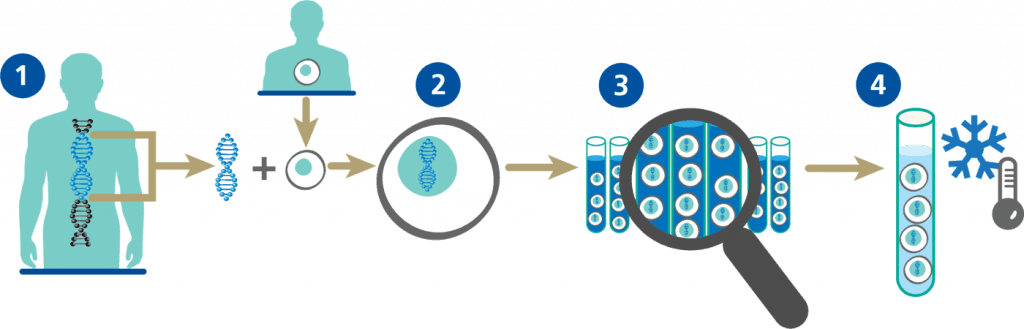
NUWIQ Production
Initial steps using recombinant DNA technology to create NUWIQ production cells
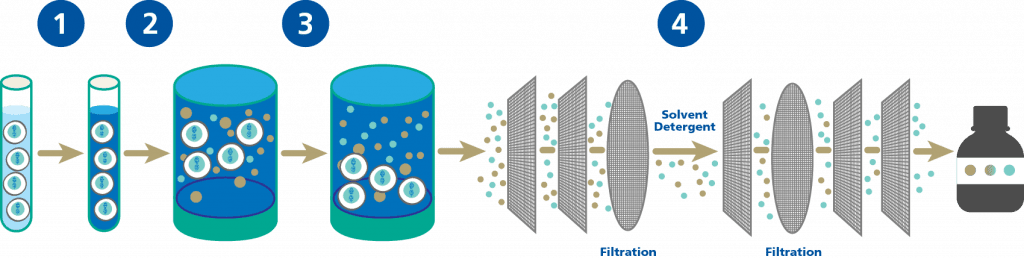
NUWIQ Purification
Ongoing steps to produce NUWIQ
and make Factor VIII
and make Factor VIII
- A solvent/detergent process to inactivate enveloped viruses (viruses that have an outer coating on them).2,3
- Nanofiltration – an advanced filtering process that can remove extremely small particles.
After purification, the recombinant Factor VIII is formulated and freeze-dried as NUWIQ
References
- Cafuir LA, Christine L. Kempton CL. Ther Adv Hematol. 2017;8(10):303–313.
- Sandberg H, et al. Thromb Res.2012;130:808-817.
- Casademunt E, et al. Eur J Haematol. 2012;89:165-176.
- Kannicht C, et al. Thromb Res. 2013;131:78-88.